The U.S. FDA can detain your imported food products at any time for any reason. Whether for sampling, testing, or detention without physical examination, when your products are detained, following FDA sampling guidelines and having an FDA sampling plan is a must.
You can choose whichever FDA import sampling and testing laboratory you wish. But, make sure the lab follows FDA sampling guidelines and can seamlessly navigate the FDA import process.
In this post, we will outline the FDA sampling plan that we use at Certified Laboratories, a Certified Group Company, to help ensure our clients' products are quickly released from FDA detention.
Expert FDA Sampling Plan & Testing per FDA Sampling Guidelines
The FDA is specific about what it is looking for. Whether routine, random, or for-cause sampling and testing, they are seeking answers about imported products that only accredited laboratories can provide. When an FDA Notice of Action is received, our experts verify…
- Entry documents
- Lines
- Lots
- Product codes to be sampled and tested
- Product location and packaging
- Other specific information FDA wants
Next, we develop a sampling plan based on FDA sampling guidelines and upload the complete package of information into our proprietary Labware system. After generating collection reports and appropriate sample labels, our Samplers are dispatched to the detention warehouse.
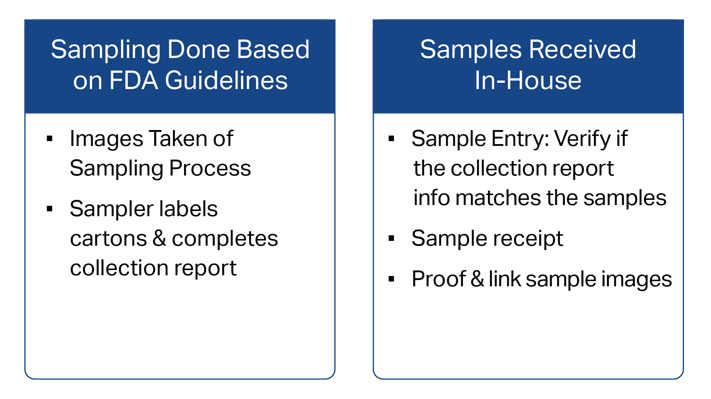
Follow FDA Sampling Guidelines
The FDA needs verification that your detained product’s sampling and testing is conducted in accordance with their defined FDA sampling guidelines.
By following these guidelines, FDA will have a complete picture of the product in question. Typically, following FDA sampling guidelines translates into samples taken at various locations within the packaged shipment, with cross-referencing against photographic confirmation of appropriately labeled cartons and collection points.
Your samples will be retained and documented in our strict chain of custody, providing confidence that what you shipped is what is sampled and tested.
Expedited Testing
Once the samples are received in our laboratories, our scientists expedite the FDA-required tests in our ISO 17025-accredited laboratories.
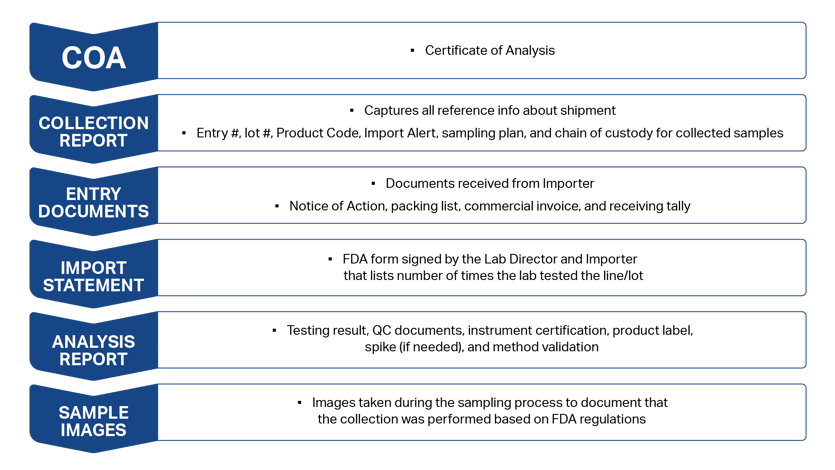
Once results are returned, a Certificate of Analysis (CoA) is generated based on the collection report that captures all of the shipment reference information, including line and lot numbers and how many tests were run.
Prior to submitting the information to the FDA, the Lab Director and the importer verify the following:
- Test results
- Quality control validation and verification
- Instrument certifications
- Product labels
- All other necessary items
- Method validation documentation and any spikes, if required
We Send Submissions Through FDA ITACS
The FDA requires electronic document submissions, and our proprietary Labware software speaks directly to the Import Trade Auxiliary Communication System, known as FDA ITACS. We send required documentation to the FDA with a copy to the importer, ensuring both parties have a complete and accurate picture of test results.
We support thousands of clients each year, helping them understand expectations regarding FDA sampling guidelines as well as expedite testing of their detained products in support of US entry.
Regulatory Support When You Need It
In addition to FDA sampling and testing, our regulatory consulting arm, EAS Consulting Group, facilitates import entry through U.S. Agent services, regulatory assistance with detained products, and more.
With more than 200 former FDA and industry leaders, our regulatory team has experience with the Foreign Supplier Verification Program (FSVP), provides assistance with the expedited entry Voluntary Qualified Individual Program (VQIP), and acts as a Qualified Individual for importers of food into the U.S.
Response times vary when it comes to FDA sampling plans and testing programs, and so does expertise. When you receive an FDA Notice of Action, you need to know that your FDA testing laboratory is working diligently and quickly for you.
Contact Certified Laboratories now to get help with FDA sampling and testing of your detained products .
