Everyone wants to cut in front of the line, especially when your company's time and money depend on importing shipments as quickly and smoothly as possible. The FDA's Import Trade Auxiliary Communications System (ITACS) allows importers to do that.
FDA says it prioritizes review of documents submitted through ITACS over those submitted by other means. In this post, we'll answer a few key questions, like "What is ITACS?" and "How do I get an ITACS account?"
What is ITACS?
"ITACS" is yet another acronym is an industry filled with them. So, what does ITACS mean?
ITACS = Import Trade Auxiliary Communications System
FDA introduced the system to improve communication with the import trade community. The Agency encourages importers and others in the industry to use the system to check the entry submission status of a shipment, receive Notices of Action electronically (i.e. faster) and to realize other benefits we'll talk about below.

Import documents submitted through the FDA ITACS system are given priority for review, helping importers get their shipments into the United States faster.
FDA ITACS Basic Functionality and Account Management
Here's where things can get a little confusing. FDA ITACS provides two levels of functionality. It's helpful to think of them as "tiers of service", with the upper tier providing additional functionality and benefits.
ITACS Basic Functionality
As the name suggests, ITACS Basic Functionality, introduced in 2011, provides limited benefits. However, it does not require users to maintain an account or enter login information. All that's needed to use the service is a valid Customs entry number that you've transmitted to FDA.
Use ITACS basic functionality here.
4 Abilities of FDA ITACS Basic Functionality
- Check the status of FDA-regulated entries and lines.
- Submit entry documentation electronically.
- Electronically submit the location of goods availability for those lines targeted for FDA examination.
- Check the estimated laboratory analysis completion date for sampled lines.
You can submit files as PDFs, as well as other common file types, such as Microsoft Word or .jpeg. And, remember, FDA prioritizes review of documents submitted through ITACS, which is a major benefit in an industry where speed is crucial. That's one reason nearly 1 million documents were submitted through ITACS in 2022.
ITACS Account Management
That brings us to ITACS Account Management functionality, which provides these additional abilities compared to Basic Functionality:
- Enables the electronic distribution of Notices of FDA Action via email and as downloads from within ITACS.
- Allows account holders to view the details of specific information requests, which are currently delivered via hard-copy Notices of FDA Action.
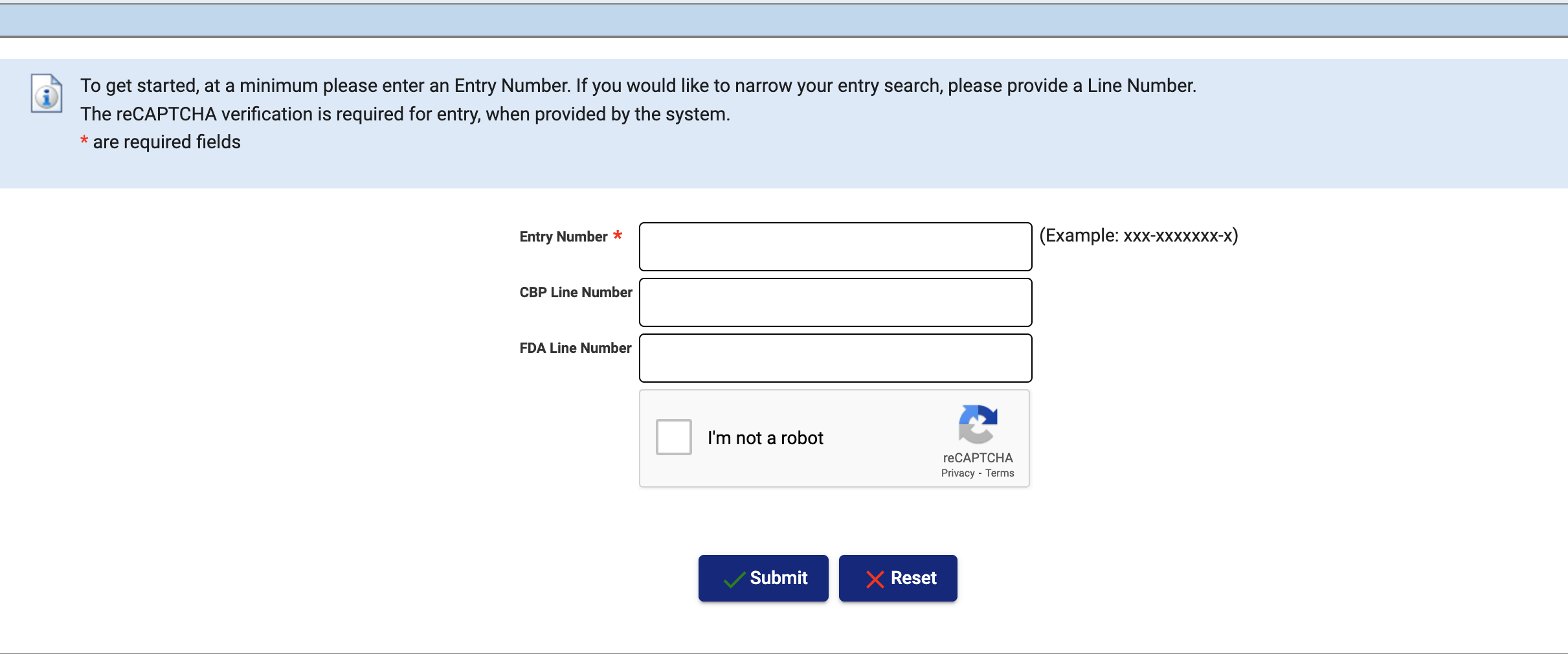
FDA ITACS basic functionality does not require a username or password. All you need is the entry number to get started.
How Do I Get an FDA ITACS Account?
As said, an account is not required to use ITACS Basic Functionality. Just head to the ITACS portal with a valid Entry Number.
ITACS Account Management, however, is reserved for Filers, Importers of Record, and Consignees with an approved ITACS account. A firm must have been a party to a previously transmitted, non-disclaimed FDA entry to be granted an account.
ITACS accounts are limited to one person in the firm at the corporate level. This person should be a high-ranking individual within the firm since they will be responsible for creating and managing ITACS accounts for other users within the firm.
Request an ITACS account here.
FDA ITACS Account Approval Typically Takes 2-3 Business Days
If FDA grants your request, you can upload documents to ITACS through your account portal, helping speed the import process. It's important to note that you don't need an account to import FDA-regulated goods, but it is an important tool in your arsenal to help shipments move as quickly and smoothly as possible.
ITACS account approval usually takes 2-3 business days, and potentially longer if FDA requests additional information.
How the Benefits of ITACS Help You
Whether FDA ITACS Basic Functionality suits your needs, or you decide to request an ITACS account, the system improves communication and helps you get the information you need to navigate imports as quickly as possible. And time is money in this business. According to FDA, ITACS benefits include the following:
- More detailed FDA entry/line statuses than what is currently transmitted to filers via CBP systems, reducing the need for phone calls inquiring about entry status.
- Elimination of the need to email or fax entry documentation and goods availability to FDA.
- Elimination of problems with lost documents.
Additional benefits of ITACS Account Management include…
- Faster receipt of Notices of FDA Action via email or download in ITACS.
- No need to maintain paper copies of Notices of FDA Action as they will continue to be available in ITACS even after an entry is closed.
- Faster receipt of requests for specific information by email or ITACS.
Navigating the challenges of U.S. imports can be tough on your own. Our personnel are well-versed with the FDA ITACS system and have plenty of experience uploading the correct documentation directly into the system for clients. Contact our FDA import and detention experts if you have questions or need help.


