Every food manufacturer wants to make a safe product. Scan the website of nearly any grower, processor, or manufacturer, and chances are the words “safe” and “high-quality” appear somewhere.
Almonds, other tree nuts, and spices are no exception, which is why it is important to conduct studies to verify that your processes can produce products safely. A process validation is a key tool in your arsenal.
In this post, we will reveal how to prepare for a process validation for almonds (most steps apply to other tree nuts, spices, and other products, too).
What is Process Validation?
You are a grower, handler, or manufacturer, and you have invested the time and money into safely producing the products that bear your label. How do you gather the data needed to demonstrate that your process is efficacious? This is where process validation enters the picture.
Process validation is the practice of obtaining data to support the effectiveness of your process in achieving its desired outcome – namely, almonds that are free of Salmonella and safe to eat.
Most often, process validation for almonds is a comparison of initial organism concentration (typically using surrogates) with organism concentration after the pasteurization process is complete (for example, a 5 log reduction kill step of the surrogate organism).
The purpose of process validation for almonds is typically to ensure a minimum 4 log reduction in Salmonella bacteria.
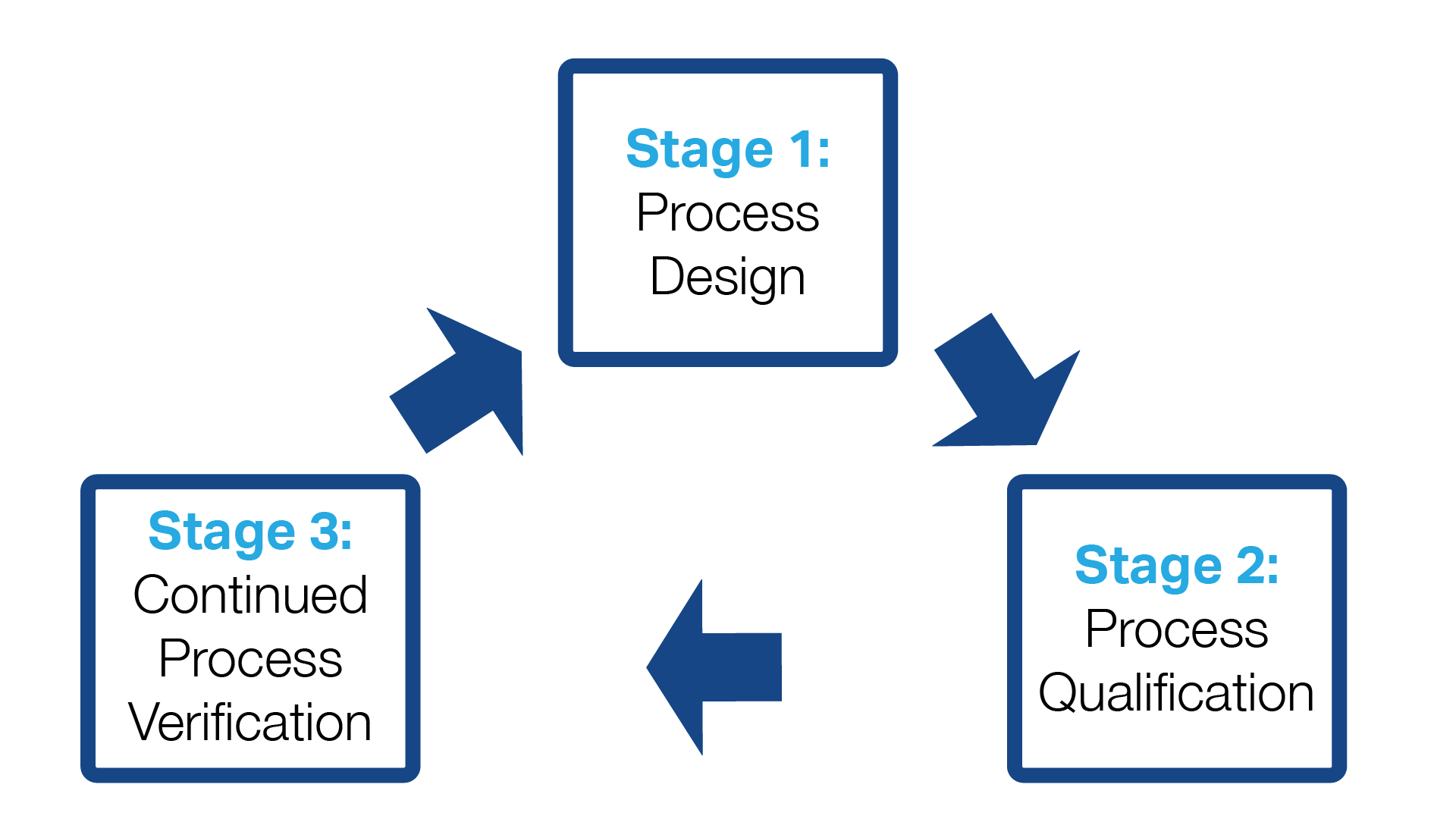
Process Validation for Almonds Includes 3 Stages
It is helpful to think of process validation for almonds (and other products) as, indeed, a multi-stage process rather than a one-and-done event. That means your involvement continues after the process validation has been completed and you have received a report from the Process Authority.
Think of it as a continuous feedback loop during which data is collected, analyzed, and used to finetune your process to maximize food safety. The data may require you to make minor or major alterations to the process.
This is known as process verification, and it is vital for confirming that critical parameters have been implemented and verified. Take a deeper dive into process validation here.
Why Perform Process Validation for Almonds?
- In the United States, the Code of Federal Regulations (7 CFR 981) requires almond handlers to subject their almonds to a process that achieves a minimum 4-log reduction in Salmonella bacteria prior to being shipped.
- Customers demand safe products, whether it is a large retailer with whom you are working or people who buy your almonds directly from your website.
- Your company’s quality-control program can benefit from a process validation. QC personnel are tasked with ensuring products are produced safely to protect customers and your brand. A recall can cost millions of dollars, not to mention brand damage that can take years to repair.
Salmonella Outbreaks Drive Need for Pasteurization and Validation
The mandate to pasteurize all almonds in the U.S. and Canada began with a Salmonella outbreak in Canada in 2001. It was traced to raw almonds sold in bulk bins that originated from three different orchards in California. The Almond Board of California (ABC) initiated an educational program to help almond growers and handlers adopt Good Agricultural Practices (GAPs), Good Manufacturing Practices (GMPs) and Sanitation Standard Operating Procedures (SSOPs) to help safeguard consumers.
But, in the spring of 2004, another Salmonella outbreak occurred, this time in Oregon. It was linked to raw almonds provided by a single handler. The handler voluntarily recalled 5 million pounds of almonds sold in the United States. The recall eventually encompassed 15 million pounds of almonds that had been exported to eight different countries.

Salmonella outbreaks in 2001 and 2004 traced to raw almonds triggered the mandate to pasteurize all almonds sold in the U.S. and Canada.
Almond Board of California Takes Action Against Salmonella
That summer, ABC approved a voluntary plan that recommended treatment for all almonds to fight Salmonella.
Two years later, in 2006, the Board recommended implementation of a mandatory program that requires all almond handlers to subject their almonds to a process that achieves at least a 4-log reduction in Salmonella before they can be shipped. A 4-log reduction means bacteria has been decreased by a factor of 10,000 (four zeroes).
What are the Different Treatment Processes for Almonds?
There are multiple treatment processes for killing Salmonella bacteria on almonds. Whichever is used, it must incorporate technologies that have been shown to achieve a minimum 4-log reduction of Salmonella bacteria pursuant to a Letter of Determination from FDA or approval by the Almond Board of California’s Technical Expert Review Panel (TERP).
At this point, FDA has issued such Letters of Determination for the following treatment processes for almonds:
- Oil roasting
- Blanching
- Propylene oxide (PPO)
- Moist heat (steam) processing
Neither FDA nor TERP recommend a specific process; rather regulatory oversight ensures whatever process is chosen meets scientific criteria to consistently achieve the required minimum 4-log reduction of Salmonella bacteria.

Oil or dry roasting are just two methods of killing Salmonella bacteria on almonds. Whichever method you choose, it must consistently achieve a minimum 4-log reduction of Salmonella.
There are multiple processes handlers can use depending on the desired sensory and nutritional qualities of the finished product, so they need to keep that in mind when choosing a treatment process for almonds. Here is a closer look at some of them:
- Oil roasting: Almonds are exposed to hot oil at 260°F or higher for a minimum of two minutes. A University of California at Davis study showed that oil roasting will achieve a 5-log reduction kill step of Salmonella. See ABC’s Guidelines for Validation of Oil Roasting Processes.
- Blanching: Almonds are exposed to hot water at 190°F or higher for a minimum of two minutes. A University of California at Davis study showed that blanching will achieve a 5-log reduction kill step of Salmonella. See ABC’s Guidelines for Validation of Blanching Processes.
- Propylene oxide (PPO): Almonds are treated with propylene oxide for four hours to achieve a 5-log reduction kill step. PPO is a registered fumigant in the U.S. for reduction of bacteria, yeasts, and mold on raw nut meats. Most export countries do not accept this method, so it is used infrequently. See ABC’s Guidelines for Validation of Propylene Oxide Pasteurization.
- Moist heat (steam) processing: Almonds are subjected to steam and high-heat dehydration, during which they reach a minimum 220°F to achieve the minimum 4-log reduction kill step of Salmonella. The process can vary depending on the handler, but ABC’s Technical Expert Review Panel has reviewed and accepted two proprietary processes used by two different handlers at time of writing. ABC provides guidelines for conducting a process validation for almonds using steam here.
- Dry roasting: Dry roasting involves subjecting almonds to dry air heated to a range of 265°F to 310°F for 15-30 minutes, depending on the efficiency of the roaster, to achieve a 4-log reduction kill step. The Almond Board of California published Guidelines for Validation of Dry Roasting Processes that outlines steps the Process Authority should take when conducting a process validation for almonds using the dry roasting process.
Who Can Perform a Process Validation for Almonds?
Federal regulations (7 CFR 981) require handlers to use only treatment processes for almonds that have been validated by a Process Authority approved by the Almond Board of California.
A Board-approved Process Authority has expert knowledge of ABC-validated treatment processes for almonds and is qualified to conduct a process validation. A Process Authority must also have additional knowledge to be approved by the Board, including the following:
- An understanding of the equipment used in the treatment processes for almonds.
- Experience conducting studies to determine the ability of the equipment to achieve the required 4-log reduction kill step.
- Knowledge of when sufficient data has been gathered to ensure the quality of the final product.
A Process Authority may also establish a new treatment process for an almond handler, which is distinguished from validating an existing treatment process. Either way, the process must always achieve the required minimum 4-log reduction of Salmonella.
Currently, there are only about 15 ABC-approved Process Authorities. Two of them are based in Turlock, California with Certified Laboratories, a Certified Group Company, and have been supporting the California almond and tree nut industry for 15 years.
How Long Does a Process Validation for Almonds Take?
While the Process Authority’s work at the processing facility will typically take 1-2 days, designing, preparing, and conducting a process validation for almonds is a big undertaking that can take several weeks from start to finish.
For example, a minimum of 10 days is required to prepare surrogate organisms for the process validation. In addition, it normally takes 3-4 weeks to receive a validation letter from the Almond Board of California after all data has been submitted.
Understanding the process validation and preparing will help it go as smoothly and quickly as possible. The steps below will help you prepare. You can also download our Process Validation Checklist to help prepare you for a process validation for almonds.
Process Validation for Almonds: What to Expect
Certified Laboratories has a 5-step process for conducting a process validation.
Step 1: Pre-Validation Preparation
- Initial client contact: Discuss the scope of the project with the Process Authority and receive an Equipment Form to complete.
- Define the reasons for the process validation: Regulatory requirement, customer requirement, etc.
- Identify the product matrices and processes to be validated.
- Define the process parameters: Is this a new process or piece of equipment?
- Set the raw material specifications: Determine the worst-case scenario for the raw product to be processed.
- Pre-validation surrogates: The Process Authority will make recommendations regarding use of surrogates.
- Validation timeline: Establish a tentative date for the validation.
Step 2: Pre-Validation Quote
- The Process Authority will provide a written quotation that outlines project cost and deliverables.
- Upon approval, the client will provide treated product for inoculation (except skin-on almonds, which Certified Laboratories will provide).
- The client will receive a validation checklist that includes activities that must be completed three weeks prior to the validation taking place. This ensures you are ready when the PA arrives.
Step 3: Pre-Validation Final Preparation
- Validation readiness: The Process Authority has a final discussion with the client to verify all checklist items have been completed. This helps ensure everything goes smoothly and is completed in a timely manner.
- Validation date: Confirm the validation date and schedule surrogate preparation. Since preparation takes a least 10 days, it is highly recommended that product be sent to the lab well in advance of the trial date to avoid rush shipping charges or delays.
Step 4: Validation
- Surrogate samples: Inoculated surrogate samples will arrive at the facility 1-2 days prior to the validation. Make sure to handle and store them according to the provided instructions.
- Process authority: The PA will arrive at the facility on the validation date. Please have the following items ready:
- Small table
- Chair
- Access to an electrical outlet
- Paper towels
- The surrogate samples
- The cooler/ice packs
- Raw material specifications: Temperature and moisture of the input product will be verified along with required quantity.
- Equipment evaluation: Measurements and details of the equipment will be recorded for the validation report. Process parameters will also be verified and recorded.
- Surrogate placement and retrieval: The surrogates and/or temperature recording devices will be placed and recorded, along with time of retrieval.
- Post processing contamination evaluation: The PA will evaluate the potential re-contamination of treated product by evaluating plant design and the environmental monitoring program.
Step 5: Validation Report
- A written report will be provided for validations that achieve the desired log reduction.
- Evaluation of the treated surrogate enumeration results along with the process data will follow completion of the on-site validation.
- For products other than almonds, the final validation report is typically completed two weeks after validation completion.
- For process validations for almonds, the PA will submit the validation report to the Almond Board of California for TERP approval. After TERP reviews the report, the approval may be granted, but generally TERP poses additional questions regarding the validation. TERP’s initial questions typically arrive two weeks after the report submission and the PA will respond in writing addressing any concerns. If the response is satisfactory, TERP will issue the validation certificate. TERP approval is typically received 3-4 weeks after report submission.
Why Choose Certified Laboratories for Your Process Validation for Almonds, Nuts & Spices?
Process validations are as different as the products and processes being evaluated. Expertise evaluating one product or treatment process does not necessarily translate into expertise evaluating your products or processes. Certified Laboratories has expertise with process validations in multiple industries, including almonds, tree nuts, and spices.
- Almond Board of California-approved Process Authorities with 15 years of experience supporting the almond industry.
- We draft our validation reports to meet regulatory expectations in the event of an audit or recall.
- FDA historically accepts our validation reports when performing an audit.
- Local support with a testing laboratory and Process Authorities based in Turlock, California.
- Full range of microbiological testing for tree nuts and chemistry testing with fast turnaround times and flexible pick-up schedules.
If you have questions about conducting a process validation for almonds, other nuts, or spices, provide a few details about your needs and we’ll be glad to help.

