Reviewed and Approved by Pamela Galarza-Goedtel, Supervisor of Food Forensics, Certified Laboratories
1-Minute Summary
- There are three primary methods for analyzing and identifying filth, extraneous material, and foreign matter in food and beverage samples: Light Filth Extraction, Sanitation, and Foreign Matter Identification.
- FDA Defect Action Levels (DALs) are closely aligned with Light Filth Extraction and Sanitation.
- Common contaminants on the FDA Food Defect Levels list include mold, rodent filth, insect fragments, and foreign matter.
- Most Foreign Matter Identification analyses are the result of consumer complaints or QC inspections of finished products.
- Product testing frequency depends on risk, supply chain, and manufacturing processes.
Why Perform Food Forensics & Filth Testing?
Regardless of their origin, no one wants filth or extraneous materials in their food. Not only can it be unsafe, it’s a public relations nightmare if a customer bites into an insect, chunk of metal, or other objectionable object. For that reason, food forensics and filth testing are important to your quality control program.
In this article, we’ll explain the methods our scientists use to isolate filth elements and identify foreign materials in samples. We’ll also reveal some of the worst objects we’ve found during Foreign Matter Identification analyses.
Lastly, we’ll explain an important aspect of filth testing, namely FDA Defect Actions Levels as outlined in the FDA Food Defect Levels Handbook.
Let’s get started.
20 of the Worst Things We’ve Found in Food & Beverages




















Food Forensics & Filth Testing Explained
We know that food forensics and filth testing are important to your QC program. So, how is it performed?
There are three primary methods for analyzing and identifying filth, extraneous material, and foreign matter in food and beverage samples. Each method is tailored to the complexity of the matrices being analyzed and includes…
|
Method |
Instrumentation |
Matrix |
Materials of Interest |
|
Light Filth Extraction |
Microscopic |
Ground spices & similar complex matrices |
Insect fragments, animal hairs, feather barbules, mites, etc. |
|
Sanitation |
Macroscopic (naked eye) |
Whole products (e.g. rice, black peppercorns, whole dried fruit) |
Whole insects, excreta, mold, insect defilings, etc. |
|
Foreign Matter Identification |
Fourier Transform Infrared (FT-IR) spectroscopy |
Finished products |
Plastic, metal, rocks, glass, rodents, etc. |
Light Filth Extraction
Light Filth Extraction is generally run on complex matrices, like ground spices. In this case, “filth” can be best described as insect fragments, animal hairs, and other extraneous materials such as feather barbules and mites. Due to the small sizes, filth elements must be viewed under a microscope.

Sanitation analysis is ideal for finding macroscopic extraneous matter, like mammalian excreta, in whole products, such as black peppercorn, rice, or dried fruit.
Sanitation
Sanitation analysis is run on whole products such as rice, black peppercorn and other whole spices, and whole dried fruit. In this examination, “filth” can be best described as the extraneous material left behind by contaminants of the products.
These include whole insects, mammalian excreta, mold, and insect defiling, as well as any extraneous materials such as plastic, rocks, metal, glass, etc. These elements are examined by the naked eye.

Foreign Matter Identification, typically the result of a consumer complaint or internal QC inspection of a finished product, is used to fully identify the foreign object, often via FT-IR analysis.
Foreign Matter Identification
A Foreign Matter Identification analysis is run when an unknown object (plastic, metal, rocks, glass, rodents, etc.) other than the sample is isolated and needs to be specifically identified and its origin determined. This is different than extraneous matter because this foreign matter is fully identified using Fourier Transform Infrared (FT-IR) spectroscopy.
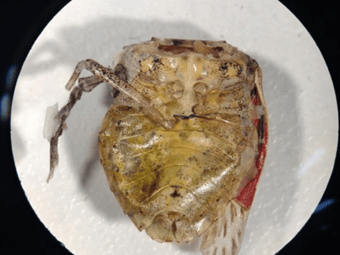
Identification of foreign matter (such as the examples in the images in the slideshow above) is typically performed on samples that originate as complaints from retail consumers (legal/insurance cases) and from internal QC physical inspections of finished product.
What are FDA Food Defect Levels?
That brings us to FDA Defect Action Levels (DALs), which are closely aligned with Light Filth Extraction and Sanitation analyses.
Defect Action Levels are regulatory guidelines established by the U.S. FDA with the purpose of protecting the public health from harmful substances or conditions in food products.
When a food product exceeds a Defect Action Level, it may be considered "adulterated" and subject to enforcement actions from the FDA or other government agencies.
What are Common Contaminants on the FDA Food Defect Levels List?
A broad range of contaminants appears on the FDA's Defect Action Levels list. Here are some of the most common:
- Mold: Sometimes found in apple butter, canned fruits, and tomato paste, mold can occur due to postharvest infection or improper storage conditions.
- Rodent Filth: This can include rodent hair or droppings, which are sometimes found in spices, grains, or other dry food products.
- Insect Fragments: Insects or their parts can be found in a wide range of products, including chocolate, peanut butter, and wheat.
- Foreign Matter: This refers to any extraneous material not normally found in food, such as glass, metal, or plastic.
- Drosophila Fly: These small flies are often found in fruit products, especially those with high sugar content.
- Pits/Stones: These can be found in products such as cherries, peaches, or olives where the pit or stone should have been removed.
- Parasites: Parasites such as roundworms can be found in seafood, particularly in fish.
- Decomposed Material: This could include decomposed pieces of the product itself, such as rotting fruit in a fruit-based product.
- Mites: These tiny insects can be found in grain-based products like flour or cereal.
- Mammalian Excreta: This term refers to fecal matter from mammals, which can sometimes be found in grain products.
Insect fragments are one of several contaminants included on the FDA Defect Action Levels list.
What are the FDA Defect Action Levels for Some Common Foods?
The FDA Food Defect Levels Handbook provides a comprehensive list of the various food defects that may pose a hazard to human health. Here are examples of just a few:
|
Product |
Defect |
Defect Action Level |
|
Canned Peaches |
Mold/Insect Damage |
Average of 3% or more fruit by count are wormy or moldy. |
|
Ground Cinnamon |
Insect Filth |
An average of 400 or more insect fragments per 50 grams. |
|
Wheat Flour |
Rodent Filth |
Average of 1 or more rodent hairs per 50 grams. |
|
Tomato Paste |
Mold |
Average mold count in 6 subsamples is 45% or more and the mold counts of all the subsamples are more than 40%. |
|
Macaroni & Noodle Products |
Rodent Filth |
An average of 4.5 rodent hairs or more per 225 grams in 6 or more subsamples. |
What is the Purpose of FDA Food Defect Levels?
The FDA uses Defect Action Levels primarily as guidance rather than enforceable regulations. The levels serve as thresholds indicating what the FDA considers to be an unacceptable amount of specific natural or unavoidable defects in food products.
If a food product is found to exceed a DAL, it does not automatically mean that the FDA will take enforcement action. However, the violation can flag the product for closer scrutiny and may trigger further actions.
FDA experts who specialize in the identification of filth and extraneous materials use a multifaceted approach to assess the significance and regulatory implications of their findings. The criteria for this evaluation are informed by specific details from the collected samples, such as the lengths of any hairs, sizes of insect fragments, the dispersion of filth throughout the sample, and the types of contaminants discovered.
Further contextualizing these findings, the FDA considers current scientific data, including the ecology of the animal species found, as well as an understanding of the product's cultivation, harvesting, and manufacturing processes. FDA Defect Action Levels serve as a useful tool for pinpointing vulnerabilities in practices related to harvesting, production, and storage.
How Often Should I Test My Products?
While the FDA does not prescribe exact testing frequencies, the Code of Federal Regulations Title 21 specifies that the manufacturer, processor, packer, and holder of food must always use quality control operations that reduce natural or unavoidable defects [21 CFR 110.110(c)]. This means that testing should be an integral part of the production process, rather than an occasional check.
Base your testing frequency and scope on several factors, including...
- Susceptibility of your products to adulteration.
- Trustworthiness of your supply chain.
- Robustness of your manufacturing processes.
- Potential risk if a product is deemed adulterated.
- Potential risk to your brand if quality slips below standards.
Performing a Food Fraud Vulnerability Assessment helps determine the appropriate frequency and scope of your testing.
Your Brand's Reputation is at Stake
While it’s virtually impossible to avoid all defects in foods, a robust QC program that includes filth testing can help you comply with FDA Defect Action Levels and protect your brand's reputation.
Contact us if you need food forensics or filth analysis for your food and beverage products.
